International Journal of Implant Dentistry – Agosto 2017 – Investigation of peri-implant tissue conditions and peri-implant tissue stability in implants placed with simultaneous augmentation procedure: a 3-year retrospective follow-up analysis of a newly developed bone level implant system
International Journal of Implant Dentistry • Agosto 2017
Indagine sulle condizioni dei tessuti perimplantari e sulla stabilità dei tessuti perimplantari in impianti posizionati con simultaneo intervento additivo: analisi retrospettiva di follow-up su 3 anni di un impianto a livello osseo di recente sviluppo
Jonas Lorenz University Hospital Frankfurt · Department of oral, maxillofacial and plastic surgery, Henriette Lerner HL DENTCLINIC, Robert A. Sader Goethe-Universität Frankfurt am Main · Center of Stomatology
and Shahram Ghanaati Goethe University of Frankfurt/Main; Universitätsmedizin der Johannes Gutenberg-Universität Mainz · Department for Oral, Craniomaxillofacial and Facial Plastic Surgery; Institute of Pathology
Lo scopo della presente analisi retrospettiva è stato di valutare le condizioni dei tessuti perimplantari e documentare la stabilità dei tessuti perimplantari in impianti C-Tech posizionati simultaneamente con un intervento additivo GBR (Guided Bone Regeneration, rigenerazione ossea guidata).
È stato analizzato clinicamente e radiologicamente un totale di 47 impianti, posizionati simultaneamente con una procedura GBR (Guided Bone Regeneration, rigenerazione ossea guidata) con un materiale osseo sintetico sostitutivo in 20 pazienti, almeno 3 anni dopo il carico.
L’analisi di follow-up ha rivelato un tasso di sopravvivenza del 100% e tassi medio-bassi di profondità di sondaggio (2,7 mm) e BOP (indice di sanguinamento) (30%). Il PES (punteggio estetica rosa) medio era di 10,1 dal valore massimo di 14.
Non erano presenti difetti perimplantari manifesti, e la perdita ossea media è stata di 0,55 mm.
In conclusione, gli impianti inseriti in combinazione con una procedura GBR (Guided Bone Regeneration, rigenerazione ossea guidata) possono raggiungere risultati funzionalmente stabili ed esteticamente soddisfacenti a lungo termine per la sostituzione di denti mancanti in caso di atrofia della cresta alveolare.
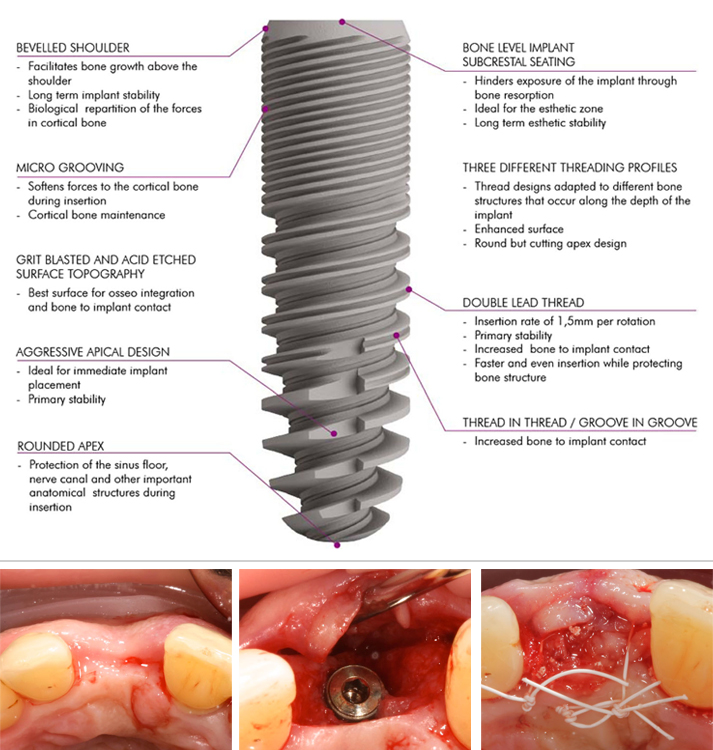
References
1. Gurgel BC, Montenegro SC, Dantas PM, Pascoal AL, Lima KC, Calderon PD. Frequency of peri-implant diseases and associated factors. Clin Oral Implants Res. 2016; doi: 10.1111/clr.12944
2. Qian J, Wennerberg A, Albrektsson T. Reasons for marginal bone loss around oral implants. Clin Implant Dent Relat Res. 2012;14(6):792–807.
3. Berglundh T, Lindhe J, Ericsson I, Marinello C, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2:81–90.
4. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the per iodontal and peri-implant tissues in the dog. J Clin Periodontol. 1999;21:189–93.
5. Moon I, Berglundh T, Abrahamsson I, Linder E, Lindhe J. The barrier between the keratinized mucosa and the dental implant. An experimental study in the dog. J Clin Periodontol. 1999;26:658–63.
6. Lindhe J, Berglundh T. The interface between the mucosa and the implant. Periodontol. 1998;17:47–54.
7. Masaki C, Nakamoto T, Mukaibo T, Kondo Y, Hosokawa R. Strategies for alveolar ridge reconstruction and preservation for implant therapy. J Prosthodont Res. 2015;59(4):220–8.
8. Damien CJ, Parsons JR. Bone graft and bone graft substitutes: areview of current technology and applications. J Appl Biomater.1991;2:187–208.
9. Cordaro L, Torsello F, Miuccio MT, di Torresanto VM, Eliopoulos D. Mandibular bone harvesting for alveolar reconstruction and implant placement: subjective and objective cross-sectional evaluation of donor and recipient site up to 4 y
ears. Clinical Oral Impl Res. 2011;22:1320–6.
10. Canullo L, Penarrocha-Oltra D, Soldini C, Mazzocco F, Penarrocha M, Covani U. Microbiological assessment of the implant-abutment interface in different connections: cross-sectional study after 5 years of functional loading. Clin Oral Implants Res. 2015;26(4):426–34.
11. Misch C. Implant design considerations for the posterior regions of the mouth. Implant Dent. 1999;8(4).
12. SteigengaJ,al-ShammariK,NocitiF,MischC,WangH.Dentalimplant design and its relationship to long-term implant success. Implant Dent. 2001;12(4):306–17.
13. Canullo L, Pace F, Coelho P, Sciubba E, Vozza I. The influence of platform switching on the biomechanical aspects of the implant-abutment system. A three dimensional finite element study. Med Oral Patol Oral Cir Bucal. 2011;16(6):852–6.
14. Lerner H, Lorenz J, Sader R, Ghanaati S. Two-year retrospective study of periimplant health and periimplant bone stability after immediate implant placement of a newly developed bone level implant system—a first report.
EDI Journal (European Association of Dental Implantologists, Teamwork Media); 2017; ahead of print.
15. Ghanaati S, Lorenz J, Obreja K, Choukroun J, Landes C, Sader R. Nanocrystalline hydroxyapatite-base d material already contributes to implant stability after 3 months: a clinical and radiologic 3-year follow-up investigation. In: Journal of Or al Implantology. 2014;40(1):103–9.
16. Lorenz J, Kubesch A, Korzinskas T, Barbeck M, Landes C, Sader R, et al. TRAP-positive multinucleated giant cells are foreign body giant cells rather than osteoclasts: results from a split-mouth study in humans. J Oral Implantol. 2015;41(6):e257–66.
17. Barbeck M, Udeabor S, Lorenz J, Schlee M, Grosse Holthaus M, Raetscho N, et al. High-temperature sintering of xenogeneic bone substitutes leads to increased multinucleated giant cell formation: in vivo and preliminary clinical results. J Oral Implantol. 2015;41(5):e212–22.
18. Barbeck M, Udeabor S, Lorenz J, Kubesch A, Choukroun J, Sader R, et al. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-Oss TM) results in an enhanced early implantation bed vascularization. Ann Maxillofac Surg. 2014;4(2):150–7.
19. Lorenz J, Barbeck M, Sader R, Russe P, Choukroun J, Kirkpatrick CJ, et al. Foreign body giant cell related encapsulation of a synthetic material three years after augmentation. J Oral Implantol. 2016;42(3):273–7.




